Scandinavian Total Ankle Replacement
Drs. Clanton and Haytmanek offer the STAR ankle replacement for individuals suffering from severe ankle arthritis.
Since the late 1800’s, severe ankle arthritis has been treated by fusing the bones of the ankle together to eliminate the arthritic joint.
This effectively treated the pain of arthritis but left the ankle joint immobile. The other large, weight-bearing joints of the body, the hip and knee, used to be treated in this same manner with a fusion, but since the 1970’s have largely been treated by joint replacement.
The procedure of joint replacement for the hip and knee has become so successful that it has been categorized as one of the most important medical breakthroughs of this century. Despite this, replacement of the ankle joint has met with less success until just recently.
As one of the investigators in this study, Dr. Clanton found the STAR ankle replacement to be very effective in treating ankle arthritis in certain patients. Dr. Roger Mann and Dr. Clanton introduced this ankle replacement procedure at Vail Valley Medical Center in late 2009.
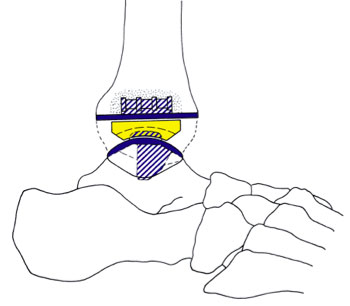
In May, 2009, the FDA approved a newly designed, three-part, cementless ankle replacement that was originally designed in Denmark and called the Scandinavian Total Ankle Replacement (STAR). (Fig. 1, A and B) This approval came after a seven-year study of the STAR ankle performed at ten institutions in the United States with over 600 patients involved.
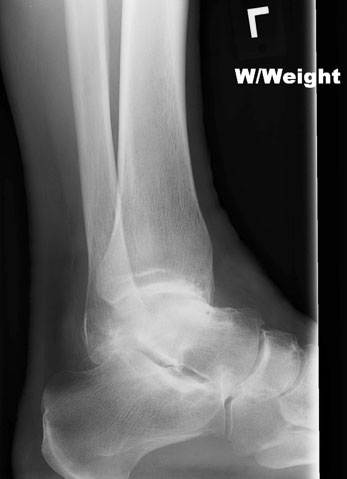
The best patients for the STAR ankle replacement are older individuals (usually above age 60) who have arthritis caused by osteoarthritis, inflammatory arthritis, or post-traumatic arthritis. (Fig. 2, A-C). There are certain conditions that make joint replacement less desirable, such as a history of joint infection, neuropathy, or severe deformity. For the younger individual with arthritis of the ankle, ankle arthroscopy or ankle fusion may be better alternatives. Newer methods involving regenerative medicine and joint resurfacing have also become available in recent years.
Those ankle arthritis patients who undergo joint replacement with a STAR can generally be expected to resume walking, hiking, road biking, skiing, and playing golf once their ankle is fully rehabilitated, but they cannot engage in high impact activities like running and jumping.
The STAR ankle replacement is one of five ankle prostheses being used in the United States today.
The other replacements are fixed bearing implants or two-part designs. They include the Agility ankle made by Depuy, the Salto Talaris ankle made by Tornier, the Zimmer ankle replacement, and the Inbone Ankle made by Wright Medical.
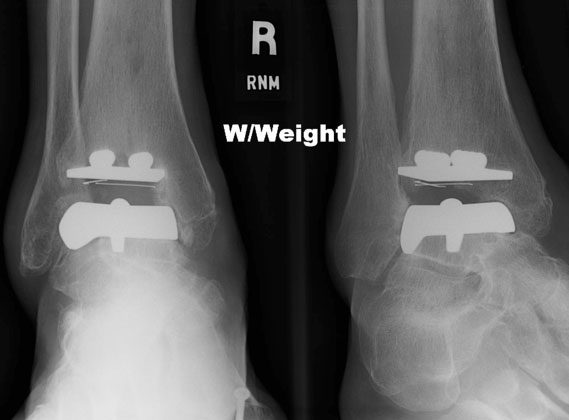
The STAR ankle replacement is the only one of the five approved by the FDA as a mobile-bearing design and for use without bone cement. It replaces the worn-out surfaces of the tibia and the talus with metal implants and a high-density polyethylene mobile bearing is placed between the metal pieces to provide a moveable surface. (Fig. 3, A-C)